We suspect that petit mal epilepsy, also known as generalized absence epilepsy, may result from a disruption of a normal sleep rhythm. The disruption results in hypersynchrony, by which we mean the massive simultaneous activity of large groups of neurons.
What then prevents the normal rhythmic activities, associated with low levels of synchrony (i.e. only small subsets of neurons participating in a population wave at any one point in time), from becoming hypersynchronous?
Our study indicates one such mechanism. Brains (mice in this case, but humans are likely
similar in this regard) seem to have built in systems to limit synchrony and thereby prevent the
onset of a seizure. See Stanford's Press Release or Science's summary ("Inhibited Rhythms") on
the findings.
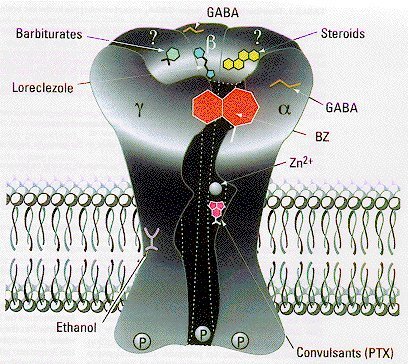
| We examined a transgenic mouse was that missing one component of the receptor for GABA (gamma-amino butyric acid). GABA is the major inhibitory neurotransmitter in the brain, and its function depends on two classes of receptors GABA-A, the major one, and GABA-B a minor class. GABA-A receptors are composite molecules, or heteromultimers (see figure at right from a review by McKernan & Whiting, 1996): they are probably composed of five different subunits proteins that combine to form a membrane pore through which chloride ions can flow through when the receptor is bound by GABA. |  |
GABA-A receptor subunits fall into several families alpha, beta, gamma, delta, epsilon and pi,
while the first three classes are likely the most important for forming functional GABA
receptors. In the brain, GABA receptors are commonly composed of two alpha, two beta, and a
gamma subunit. It is the subunit composition that determines the sensitivity of GABA receptors
to a variety of drugs, including for example, alcohol, benzodiazepines, barbiturates and some
steroids.
| In the thalamus the beta3 subunit of the beta family is largely localized to one particular nucleus (the reticular thalamic nucleus, RTN). Therefore in the beta3 knockout mouse (developed by Gregg Homanics and his team at the University of Pittsburgh) RTN neurons can't form functional GABA-A receptors. Thus synaptic inhibition between RTN neurons is nearly abolished. Note in the figure at the right that the inhibitory responses (sIPSCs) are smaller (peak), briefer (Tau D,W) and much less frequent in the knockout mice (-/-). | 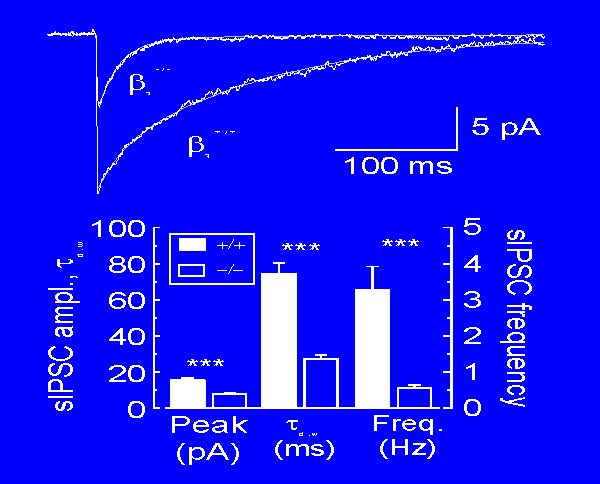 |
This is interesting because we think that the inhibitory pathways that connect RTN neurons are
important for one endogenous mechanism that prevents hypersynchrony. The thalamus is
thought to be a very important rhythm generator for both sleep and petit mal activities. This
might be better understood with an analogy: the juggers.
| Our study (Huntsman, et al. Science, 283:541-543, 1999) was in two parts. First we showed that indeed inhibitory synaptic responses were essentially abolished in (see scissors in diagram, and also the previous figure) RTN neurons, while all other neural pathways seemed to be perfectly normal. Then in the next part of the study we examined neuronal network activities in the thalamus. | 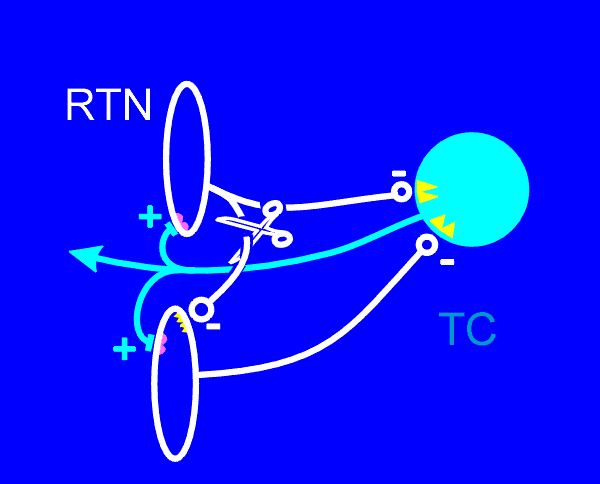 |
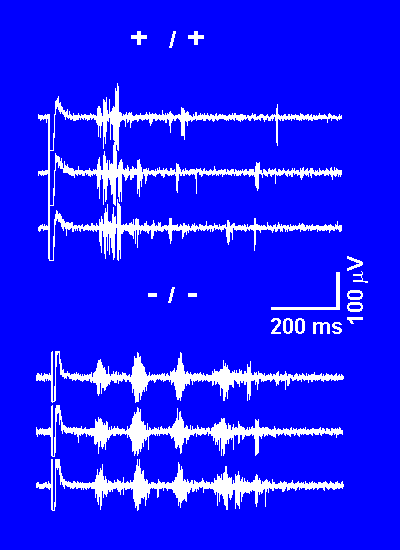 |
We found that oscillatory responses were dramatically altered in the knockout mouse. Rather than the partially synchronous responses observed in normal animals, we found that there was incredible hypersynchrony in the knockouts (-/- in the figure to the left). The responses in normal animals (+/+) were much less regular in their occurrence. These activities likely represent the semi-synchronous features of sleep-related oscillations, while the very regular and repetitive responses seen in the knockout animals are related to the generation of spike-and-wave brain activity of absence epilepsy. |
Overall, these results indicate the we can benefit from studies of the endogenous "anti-oscillatory" mechanisms in the brain. These studies will be important for a variety of epilepsies which affect over 2 million people in this country alone.